|
|
|
|
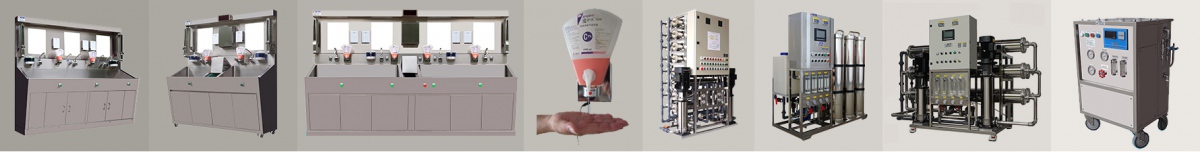
Product name : Water purification system for hemodialysis |
|
|
GMP Certificate ( base on iso-13485 requirement ) |
|
|
|
|
|
Dialysis RO System Medical License approved |
|
|
ICU single dialysis MRO- 03P |
|
|
Registration no. : 002560 |
|
|
8 ~200 beds RO-HD15 ~ RO-HF120 Dialysis RO system |
|
|
Registration no. : 002383 |
|
|
|
|
| Registration no. : 002383 | Registration no. : 002560 |
|
Product Scope : Dialysis RO system for 1~100 beds applications |
|
| Models |
Applications |
|
MRO-03P ( RO water product : 125 LPH ) |
Single dialysis RO use in ICU |
|
RO-HD15 ( RO water product : 450 LPH ) |
8 Beds Dialysis Center |
|
RO-HD20 ( RO water product : 600 LPH ) |
10 Beds Dialysis Center |
|
RO-HD30 ( RO water product : 900 LPH ) |
15 Beds Dialysis Center |
|
RO-HD40 ( RO water product : 1,200 LPH ) |
20 Beds Dialysis Center |
|
RO-HD50 ( RO water product : 1,500 LPH ) |
25 Beds Dialysis Center |
|
RO-HD60 ( RO water product : 1,800 LPH ) |
30 Beds Dialysis Center |
|
RO-HD80 ( RO water product : 2,400 LPH ) |
40 Beds Dialysis Center |
|
RO-HF30 ( RO water product : 3,000 LPH ) |
50 Beds Dialysis Center |
|
RO-HF40 ( RO water product : 4,000 LPH ) |
70 Beds Dialysis Center |
|
RO-HF50 (RO water product : 5000 LPH ) |
85 Beds Dialysis Center |
|
RO-HF60 (RO water product : 6000 LPH ) |
100 Beds Dialysis Center |
|
RO-HF80 ( RO water product : 8,000 LPH ) |
135Beds Dialysis Center |
|
RO-HF100 (RO water product : 10000 LPH ) |
165Beds Dialysis Center |
|
RO-HF120 (RO water product : 12000 LPH ) |
200Beds Dialysis Center |